
MoCRA has New, Stringent Requirements for Adverse Events :
- Display contact information on both the primary and secondary packaging
- Collect detailed personal and medical information from the consumer
- Investigate to determine if the adverse event is a “serious” event
- Report “serious” adverse event to the FDA within 15 business days
- Record all health-related adverse events and maintain records for up to 6 years
Purpose-built for cosmetic Adverse Events
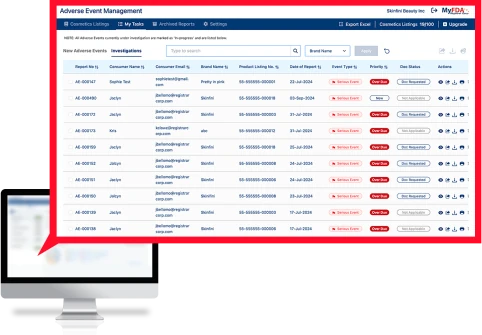
The Adverse Events Management (AEM) software securely intakes sensitive consumer medical data, tracks all adverse events for all products globally, transmits information to internal stakeholders, and formats serious adverse events to the FDA MedWatch format for submission to the FDA.




Cokion is your expert guide for Formation
Step 1 : Consumer-friendly Intake
Consumer fills digital form to confidentially submit up to 43 fields of data through web link or QR code on product label.

Step 2 : Instant Alert & Fast Response
Monitor adverse events globally for every SKU in real-time and get the earliest indicator of potential safety or quality issues.
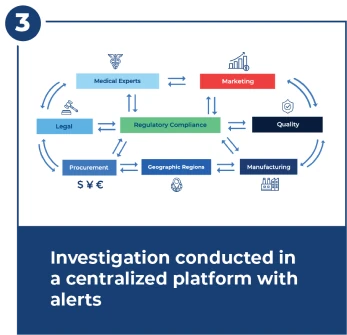
Step 3 : Investigate Within 15 Day FDA Deadline
Investigations are complex and require close and fast coordination across many teams. The investigation center provides workflows, alerts, dashboards, and centralized document management.
Real-time Alerts
Workflows
Dashboards
Document Management
Step 4 : FDA MedWatch Ready
Serious adverse event reports pre-formatted to the MedWatch 3500A for quick submission.

Step 5 : World-Class Data Security
ISO 27001-certified security for storage and transmission of medical and personal identifiable information (PII).



Your Guide to MoCRA Adverse Events
Executive Brief Covers :
- 5 key requirements for Adverse Events
- Business and legal risks of non-compliance
- Gaps in existing processes
- Recommendations for meeting requirements



